The counterfeiting of medicines is a criminal and highly irresponsible act. Every year, tons of counterfeit medicines are seized, including painkillers and medicines to treat cancer or HIV infections.4 To counteract this, the authorities and the pharmaceutical industry is increasingly working on improved counterfeit protection for pharmaceuticals.
Significant risks for patients
Counterfeit preparations may contain the wrong quantity of the active substances or may not contain any active substances at all. Falsified medicines could also be made from the wrong substances and/or contain impurities.
The counterfeit products introduced into the distribution chain pose a serious threat to the safety of patients. Taking falsified medicines can have serious consequences. If they do not contain the active substances necessary for treatment, the intended effect of the therapy cannot be achieved. Contaminated or incorrectly combined products can also trigger undesirable effects. In the worst case, the consequences can be fatal.
Greater transparency in the supply chain
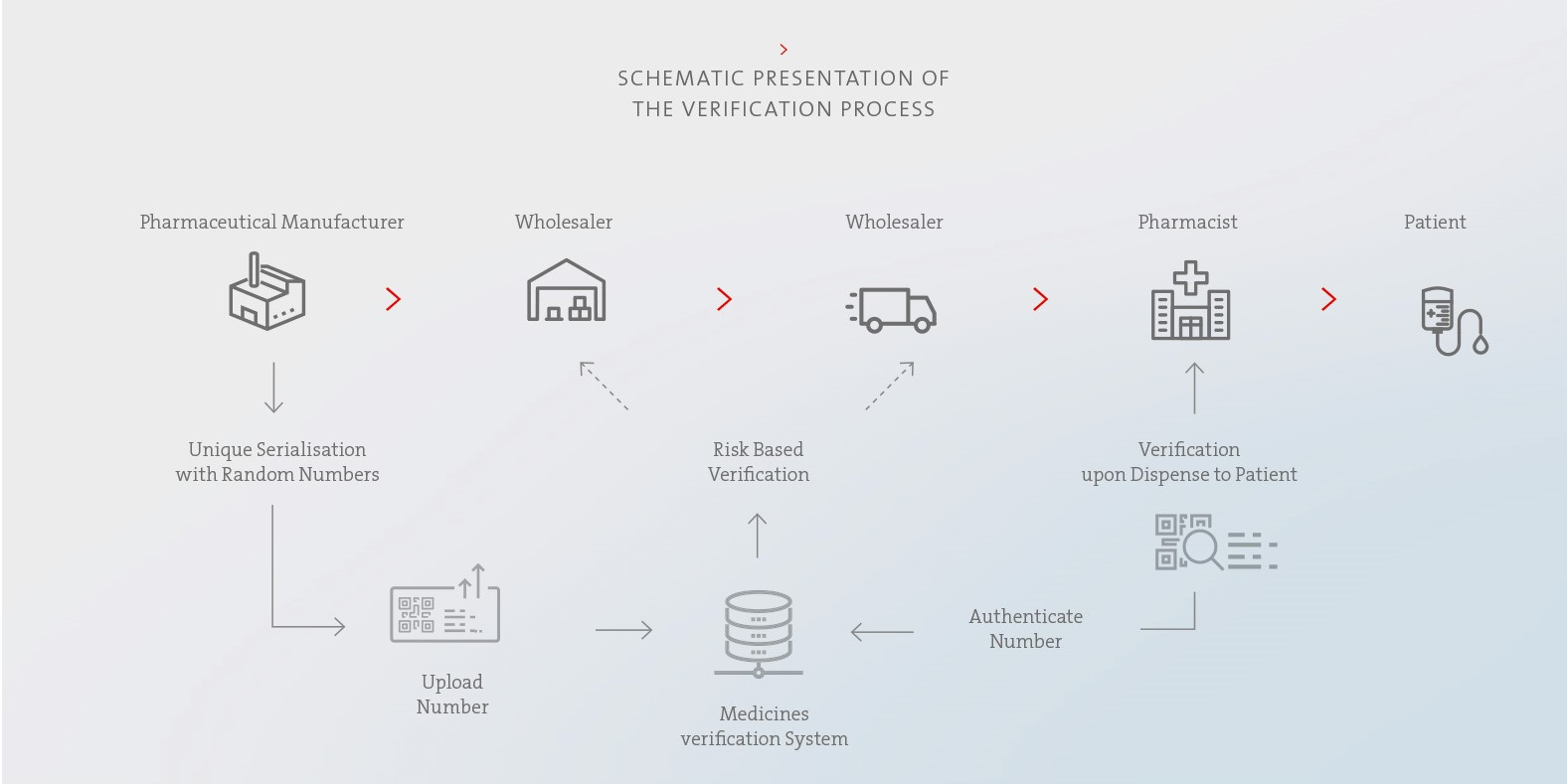
Legislators around the world have launched initiatives to curb drug counterfeiting. In the European Union, the provisions of the Falsified Medicines Directive have been in force since 9 February 2019. Key elements include the introduction of standardised security features on pharmaceutical packaging and the establishment of a verification system to implement the legally required authenticity check.
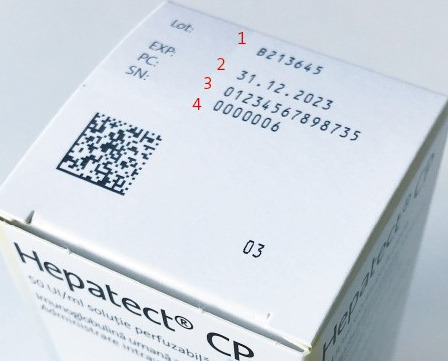
Except in exceptional cases, prescription medicines and some over-the-counter medicines may only be marketed in the European Union if they bear an individual identification mark and their integrity is easily recognisable. In order to meet this requirement, the pharmaceutical packaging is printed with a 2D barcode and issued a unique serial number. This procedure makes each individual package unique. The serial numbers assigned are stored in the national verification database of the respective EU member state. The authenticity of the preparation is checked by scanning the 2D barcode and then comparing the data with the information stored in the verification database. In the European Union, this is done immediately before the drug is administered to the end consumer, i.e. directly at the pharmacy or clinic. If the scan shows discrepancies regarding the information stored in the verification database or that the package has already been marked as delivered there, a warning message is issued and the use of a possibly counterfeit drug is prevented. In order to meet the current EU requirements for recognisability of intactness, Biotest products entering these markets have tamper-evident seals at both ends of their folding boxes as a safety feature. By testing these seals for damage, it can be ensured that only undamaged packages are delivered to the end consumer.
Concrete implementation steps for Biotest
To enable Biotest to individually check the authenticity and integrity of the preparations, our systems and processes were adapted to the requirements of the EU Falsified Medicines Directive in a multi-year project. Among other aspects, the prerequisites for the provision, handling and administration of serialisation data as well as for data transfer to the verification database were created. In today’s packaging lines, preparations are serialised by printing the serialisation data onto the folding boxes. In addition, the tamper-evident seals are applied there to document the integrity of the product.

4 500 tons of counterfeit drugs, tagesschau.de, 23 October 2018, Link: www.tagesschau.de