In research and development, compliance with all the ethical principles of drug development is our priority. Aside from efficiency, the safety of a drug is always crucial to our development efforts. In our R&D activities, we are guided by four principles:
Patient benefit
We want to develop drugs that help people affected by a serious and often chronic disease to achieve greater quality of life. Efficacy and safety are the priority. It is also important that the pharmaceutical form and dosage should be as convenient as possible for the patient and enable ease of handling.
Research where there is medical need
In the case of new developments, we concentrate on areas with a particularly high unmet medical need: diseases for which there is still no specific and promising therapy or those where approved treatments are associated with side effects.
Continuity and long-term orientation
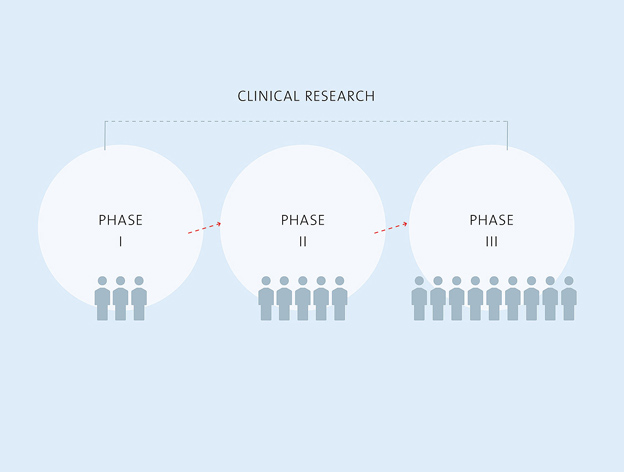
The process from drug development to marketing authorisation takes a number of years and includes numerous preclinical and clinical studies in which the development candidate must demonstrate its efficacy and tolerability. Biotest works closely with researchers and physicians in the relevant specialities and integrated study centres. Many years of trust and scientific cooperations link us with many researchers, hospitals and doctors.