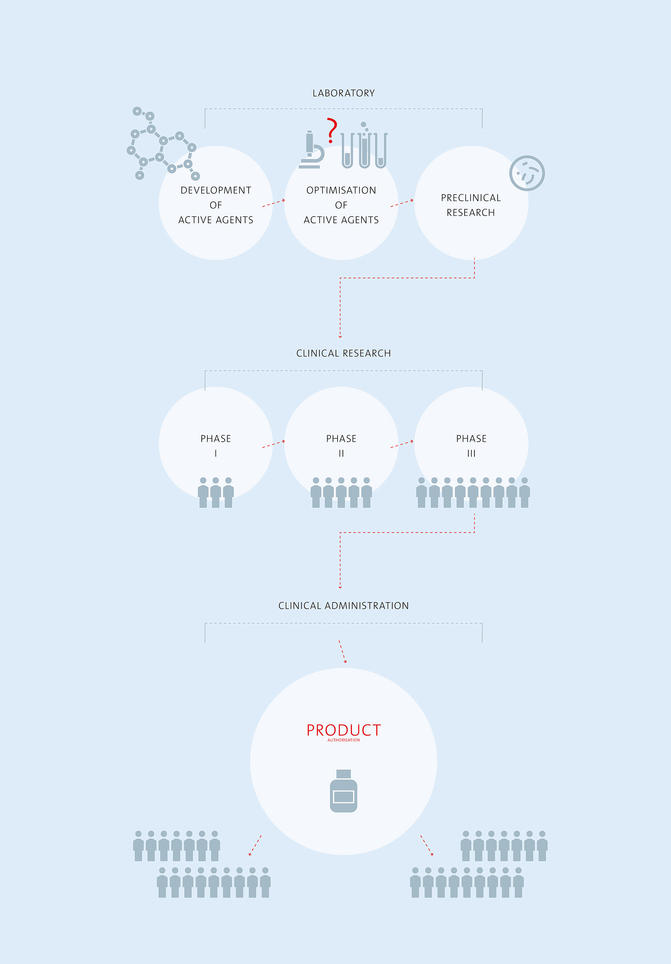
Preclinical research
In preclinical research the mechanism of action, composition and stability of the drug are tested initially in the laboratory. This is followed by tests in an animal model, which investigate whether the preparation is effective and whether side effects occur.
Phase 1
Clinical trials are divided into four phases. In phase 1 the drug is usually tested in healthy volunteers to find out how it behaves in the body; different dosages are studied.
Phase 2
In phase 2 a limited group of patients is given the preparation to find out whether it is effective and tolerated. Possible side effects should be identified and a suitable dosage has to be found. This phase can extend over two, three or more studies.
Phase 3
Phase 3 trials are randomised, controlled and prospective studies that are blinded for doctor and patient. Some of the patients receive the new medication and the others are treated with an already available therapy (comparator therapy) or are given a placebo. At the end, the results are compared.
Product Approval
Marketing authorisation usually follows the successful conclusion of phase 3. An application for marketing authorisation for a new drug can be made only when the therapeutic efficacy and safety along with a good benefit-risk relationship have been confirmed.
Phase 4
Phase 4 studies follow later to obtain additional information about safety and efficacy in large numbers of patients under ordinary conditions.